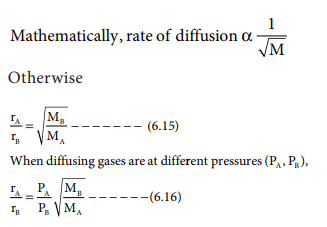
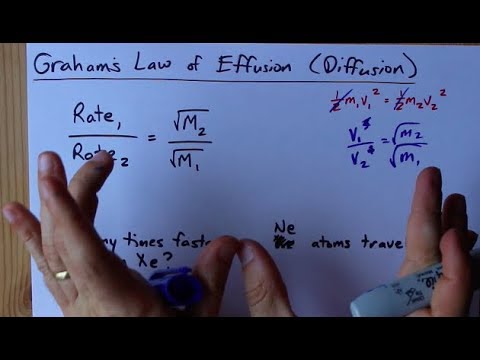
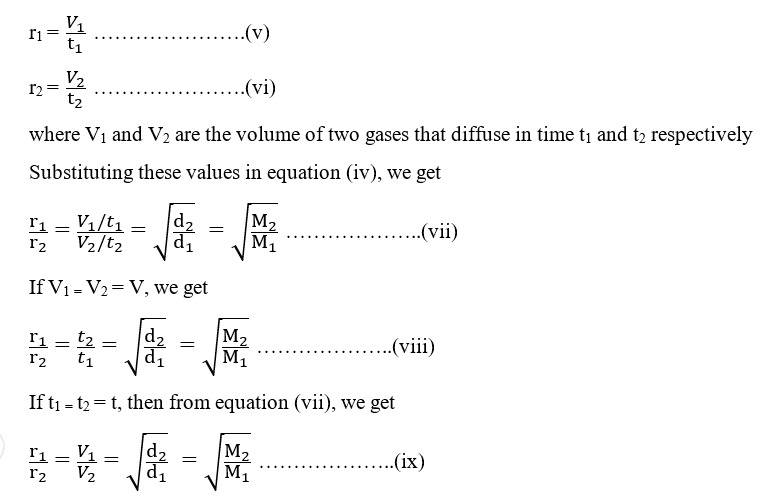Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora

Graham's Law of Diffusion vs. Effusion | Formula & Differences - Video & Lesson Transcript | Study.com
The relative rate of effusion of ch4 to so2 through the container containing ch4 and so2 in 3:2mass ratio